
Christopher Arumainayagam
Nancy Harrison Kolodny ’64 Professor of Chemistry
Links
I am a physical chemist who uses surface chemistry techniques to study radiation chemistry, photochemistry, environmental chemistry, and astrochemistry.

I am a physical chemist who employs ultrahigh vacuum (UHV) (< 1×10−9 torr) techniques such as electron stimulated desorption (ESD), temperature programmed desorption (TPD), infrared reflection absorption spectroscopy (IRAS), low-energy electron diffraction (LEED), and Auger Electron Spectroscopy (AES) to study surface chemistry, radiation chemistry, photochemistry, atmospheric chemistry, and astrochemistry. Besides teaching courses in introductory (CHEM 105, 120, and 205) and physical chemistry (CHEM 330, 331, and 335), I have taught seminar courses in surface chemistry, computational chemistry, radiation chemistry, and astrochemistry ("Chemistry of the Heavens"). I was honored to receive the Pinanski Prize and the Henry Dreyfus Teacher-Scholar Award. I collaborated with Prof Stark from the physics department to establish in 2016–2017 the Chemical Physics interdepartmental major for which I serve as the coordinator. I have been the principal investigator of four successful National Science Foundation REU summer research grant proposals (2000–2013). My research students have been awarded eight Barry Goldwater Scholarships and five Beckman Foundation Scholarships. My research has been supported by grants from the National Aeronautics and Space Administration (NASA), the National Science Foundation (NSF), the American Chemical Society (ACS), the Research Corporation, and the Camille and Henry Dreyfus Foundation. Our research has been highlighted by an American Astronomical Society(AAS) press conference, an American Chemical Society video, and a Royal Society of Chemistry article. I was the lead author of two book chapters for the Handbook of Astrochemistry, which Elsevier will publish in April 2026. In 1995, we pioneered the use of the most utilized experimental solid-state astrochemistry technique: post-irradiation TPD to identify and quantify new molecular species formed by energetic processing in astrophysical ice analogs. Our experimental and computational studies suggest that low-energy (< 20 eV) secondary electrons could be a significant contributor to the interstellar synthesis of prebiotic molecules, whose delivery by comets, meteorites, and interplanetary dust particles may have kick-started life on Earth.

Research
The core theme of my research, spanning chemistry, physics, and astronomy, remains the investigation of surface and interfacial phenomena with potential applications in various chemistry-related fields. Our recent experimental and computational work has focused on understanding the role of photochemistry and radiation chemistry in interstellar, planetary, and cometary ices. Our research was featured on the cover of Chemical Society Reviews. I am the lead author of two upcoming book chapters: “Thermal and Non-thermal Processing of Cosmic Ices” and "Experimental Techniques in Solid State and Surface Astrochemistry."
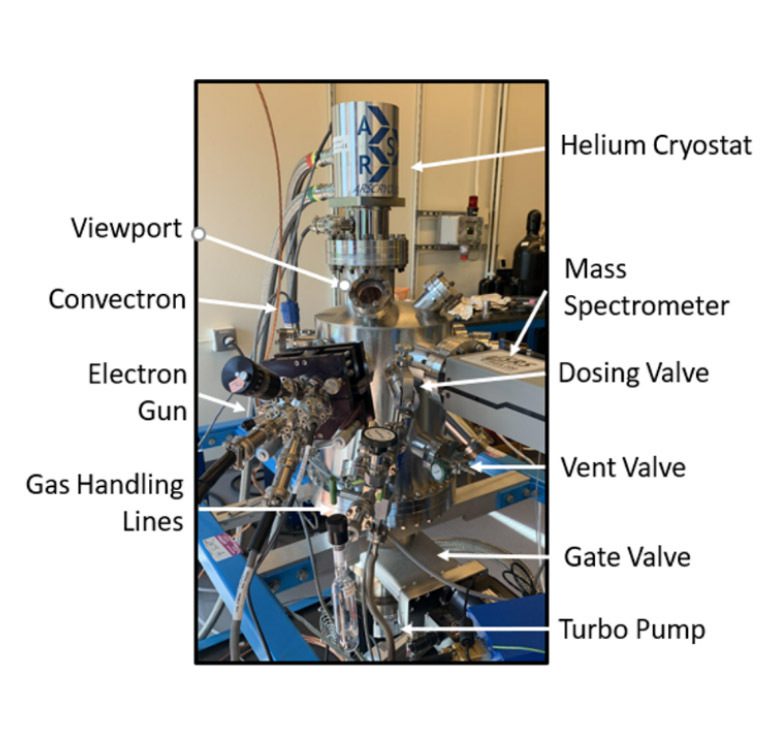
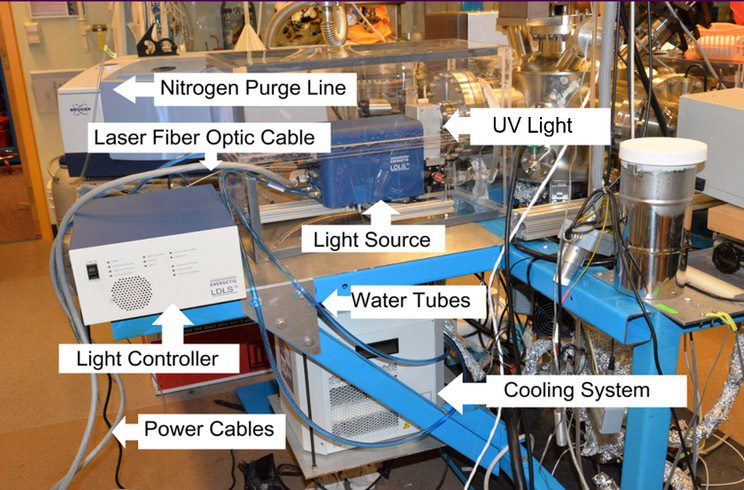
My research takes place at Wellesley College with undergraduate students, using two state-of-the-art ultrahigh vacuum chambers that feature many custom-built parts. Specialized equipment includes a laser-driven tunable low-energy (< 7.4 eV) photon source (Energetiq EQ-1500 LDLS™), a triple-filtered pulse-counting Hiden Ion Desorption Probe Series 500 quadrupole mass spectrometer (Hiden/3F RC PIC) capable of monitoring neutrals, cations, and anions in a single experiment, a closed-cycle helium cryostat (Advanced Research Systems, model DE-204PB-600), a Kimball Physics ELG-2 electron gun capable of producing 20 ns electron pulses, and a PHI FIG-5 ion source.
My students acquire practical skills such as performing helium leak checks, changing oil in vacuum pumps, building electronic circuits, spot-welding tricky junctions, and conducting complex computer modeling—abilities that are especially valuable for aspiring scientists. I have enjoyed involving numerous high school and first-year Wellesley students in my research program. Students working in my lab have presented in the Netherlands (2014), Lithuania (2016), France (2016, 2019, 2023), Chile (2017), Vietnam (2018), Italy (2020), and Spain (2024). Over the past ten years alone, more than a hundred students have conducted research in my lab, with as many as 25 working with me during some semesters.
Teaching
I see teaching and research not as two opposing forces but as a seamless combination of both. For example, I have used the equipment in my research lab to recreate the classic experiment of Davisson and Germer as a dramatic confirmation of the wave nature of matter, a concept that is highly theoretical and abstract for most students. Recently, I used my lab equipment to introduce CHEM 330 students to vacuum technology.
My teaching style involves what is now called guided inquiry. The lecture outlines (example) I hand out at the start of each lecture require students to fill in missing material, allowing them to follow along and stay engaged during the lecture. After each lecture, I also provide my students with in-depth notes (example), which require a considerable amount of my time each semester to fine-tune. What makes these notes unique is that I address student questions elicited from “one-minute” papers going back over three decades. Since 2016, I have developed numerous animated concept maps (example), which help learners follow the logical flow of ideas step by step, avoiding information overload.
Professional Activities
My external professional activities during the past five years include the Beckman Foundation Advisory Board (2021), a NASA Review Panel (2023), the Williams College Chemistry Department Visiting Committee (2022), a scholarly review of a liberal arts faculty member (2021), and a promotion review of a NASA scientist (2023).
Personal
I enjoy spending time with my wife and two children, traveling, gardening, and playing table tennis. In my younger days, I enjoyed playing cricket.
Education
- B.A., Harvard University
- Ph.D., Stanford University