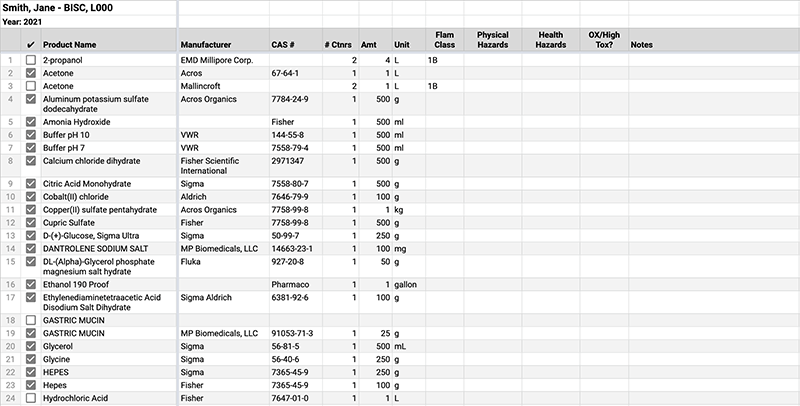
Science Center Chemical Inventories (Beta)
Guidelines for Conducting a Chemical Inventory
The first step in developing a comprehensive chemical health and safety plan is to inventory existing chemicals. This may pose significant risks to the individuals taking the inventory and ample time should be allowed to properly conduct the inventory. Serious injury can result from touching or moving chemicals that have become shock sensitive or pressurized. If any chemical container is unmarked, bulging, leaking, rusted, cracked, or has a degraded top, liquid above a solid, or crystals in a liquid, it should not be moved, even for the inventory. It is best to be cautious! Contact EHS if you have any concerns.


- If necessary, cease all other work in the laboratory while performing the inventory and allow ample time to conduct the inventory.
- Work in pairs and never work alone.
- One person should act as the recorder and the other person should list the chemicals.
- Wear appropriate personal protective equipment. This may include gloves, chemical splash goggles, a lab apron, and closed-toed shoes.
- Use a chemical cart with side rails and secondary containment.
- Use a safety step stool or a small stepladder if chemicals are stored above eye level.
- Check that chemicals have legible and appropriate labels – Abbreviations of chemical names are not appropriate as the sole indicator of a chemical's identity.
- Store flammable solvents that require storage at a reduced temperature in refrigerators or freezers designed for storage of flammable liquids. Note: Household refrigerators are not appropriate for the storage of flammable liquids.
- Check that chemicals in freezers are not frozen together or stuck to the sides or shelves. Also, check that containers are placed in secondary containment and are in an upright position.
- If kits are present, be sure to inventory all chemicals in each kit. Many older kits may contain unlabeled chemicals with only the manufacturer’s numbers on them. Record the manufacturer, the chemical number, and the size of the container, and any information concerning the manufacturer such as phone number and address as well as the kit identification number.
- If preserved specimens are present, record the preservative used. Many specimens contain formaldehyde or ethyl alcohol. (Formaldehyde is a combustible liquid; Alcohols are flammable liquids.)
- Ensure that any spills or other contamination are cleaned up properly – pay particular attention to cabinets and refrigerators.
- Discard all chemicals that are no longer useful to your research, expired (or more than 5 years old), or degraded – look for crystals, phase separation, container damage. Properly pack these chemicals and complete a Hazardous Waste Pickup request to have them removed.
- If peroxide formers are present, review Wellesley College Peroxide Former SOP